Broken bones often have to be temporarily fixed in place with screws, nails or plates to enable them to heal and grow back together again. In the past, these implants, made of stainless steel or titanium, usually had to be removed again in a second operation because this foreign material would otherwise have remained in the body where it could lead to problems (infections, bone shrinkage). Medical research has therefore been searching for many years for material capable of being naturally absorbed. Thanks to close co-operation and its major commitment to basic research, Syntellix succeeded in achieving the pioneering breakthrough with a major innovation. Previously only dreamed of by patients and doctors, this innovation has now turned these dreams into reality, and has already been used thousands of times: a biotransformable and metallic (stable) material for implants.
Syntellix AG was established in 2008 by innovation-friendly and forward-looking investors in Niedersachsen (Lower-Saxony) in Germany, under the overall management of Prof. Dr. Utz Claassen. Established in Hannover, the start-up succeeded in making the breakthrough after a great deal of highly committed work: by setting up an interdisciplinary network of outstanding scientists at various research locations, Syntellix succeeded in developing MAGNEZIX® within five years and launching it on the market - a truly revolutionary material which, despite its metallic properties and high stability, can be completely absorbed within the body - and even replaced at the same time by the body's own bone tissue.
After countless biomechanical studies, in-vitro and in-vivo tests, MAGNEZIX® was the first bioabsorbable metallic implant world-wide to receive approval in 2013 (CE label). In March 2013, Syntellix AG won the prestigious and internationally renowned German Industry Innovation Prize - first innovation prize in the world® for its unique innovation MAGNEZIX®. Only two years later, the company also won the Top Innovator of German Medium-sized Enterprises award in 2015. It won the prize again as the Top Innovator in 2016, in the same year that Syntellix also won the Future Prize of the Health Industry Club. Another milestone of the year was the state approval of MAGNEZIX® by the renowned Health Science Authority (HSA) in Singapore. In 2017 Syntellix AG won the STEP Award for the most innovative technology and the German Medical Award for the best product innovation. Also in 2017 Syntellix was awarded as Innovator of the Year by the Top 100 jury. In the following year 2018, MAGNEZIX® was named Sustainable Product of the Year at the Sustainability Awards; the German Innovation Award in gold followed in 2019. And the prizes continued in 2020: This year Syntellix was able to win the Edison Award in silver and the IMA Award of Excellence.
These numerous awards confirm the innovative strength and technological leadership of Syntellix AG. Syntellix is now the world's number 1 in the bioabsorbable metal implant sector, and currently active in more than 60 countries. Patients have already been successfully operated on ten thousands of times with Syntellix products.
History of Syntellix AG
- 2008
- 2008 - 2010
- 2010 - 2012
- March 2013
- May/June 2013
- September 2013
- January 2014
- August 2014
- January 2015
- February 2015
- March 2015
- April 2015
- May 2015
- June 2015
- July 2015
- August 2015
- September 2015
- January 2016
- February 2016
- May 2016
- June 2016
- July 2016
- August 2016
- November 2016
- December 2016
- March 2017
- April 2017
- June 2017
- November 2017
- December 2017
- January 2018
- Febuary 2018
- April 2018
- Mayo 2018
- May 2018
- July 2018
- July 2018
- September 2018
- December 2018
- January 2019
- February 2019
- March 2019
- March 2019
- March 2019
- May 2019
- August 2019
- September 2019
- September 2019
- September 2019
- September 2019
- September 2019
- October 2019
- February 2020
- February 2020
- February 2020
- March 2020
- March 2020
- April 2020
- May 2020
- October 2020
2008

ESTABLISHMENT
Syntellix was founded in 2008 with the intention to develop and pioneer the world's first degradable magnesium implant for medical use in orthopaedics, based on numerous promising results and findings from research on magnesium-based degradable implants.
2008 - 2010

MATERIAL RESEARCH AND DEVELOPMENT MAGNEZIX®
Together with new network partners which came on board (Institute for Functional Materials at the Technical University Clausthal), research was carried out in the following years on suitable materials. With the help of sophisticated mechanical, degradation and animal testing, MAGNEZIX® was finally developed, tested and optimised. MAGNEZIX® is a magnesium alloy based on the WE 43 alloy system which forms the adequate basis for bioabsorbable medical implants.
2010 - 2012

SUCCESSFUL CONCLUSION OF CLINICAL STUDIES (PROOF OF CONCEPT)
The excellent results from numerous pre-clinical studies and tests carried out during 2010 to 2012, finally led to authorisation by the ethics commission for a clinical (approval) study on human patients with MAGNEZIX® compression screws. The clinical study was successfully carried out at the Hannover Medical School (MHH).
During the course of the clinical study, the biocompatibility of MAGNEZIX® pursuant to the ISO 10993 standard was confirmed and verified by numerous tests analysing aspects such as cytotoxicity, genotoxicity, oncogenicity, systemic toxicity, and many sensitivity tests (December 2012).
March 2013

AWARD "GERMAN INDUSTRY INNOVATION PRIZE - FIRST INNOVATION PRIZE IN THE WORLD"
With its pioneering innovation MAGNEZIX® CS 3.2, Syntellix AG won the prestigious international German Industry Innovation Prize - first innovation prize in the world® in March 2013. This innovation is unmatched world-wide and generates a unique stand-alone position for Syntellix AG, and technological leadership in the metallic bioabsorbable implant sector.
May/June 2013

CE-APPROVAL MAGNEZIX® CS 3.2 FOR 30 COUNTRIES
MAGNEZIX® CS 3.2 was awarded CE-approval as a medical product by TÜV Rheinland - which meant that after five years of research and development work, the world's first magnesium-based and bio-transformable implant for use in humans was now finally available.
This certification was shortly followed by the first operations during standard clinical practise.
September 2013

MARKET LAUNCH IN GERMANY
Within a very short period of time after its market launch in Germany, more than 200 operations had already been carried out with Syntellix implants at 65 different clinics.
January 2014

EUROPEAN DISTRIBUTION OF MAGNEZIX® STARTS
The first supplies to Italy, Austria, Slovenia, as well as to Turkey, Switzerland and the Netherlands then followed.
August 2014

EXPANSION OF THE MAGNEZIX® CS PORTFOLIO
To satisfy a broader spectrum of indications, Syntellix began an R&D campaign at the beginning of August 2014 to develop MAGNEZIX® CS screws with additional thread diameters of 2.0, 2.7 and 4.8 mm.
January 2015
GLOBAL DISTRIBUTION OF MAGNEZIX® STARTS
International sales of MAGNEZIX® CS 3.2 began at the beginning of the year focussing on dynamic growth markets. Market launches in Europe in Bulgaria, Poland, Norway, Finland and Ireland then followed.
February 2015
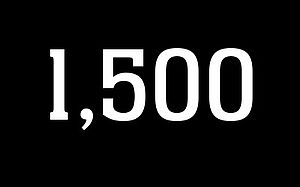
1,500 IMPLANTS
More than 1,500 implants have been successfully marketed within a span of 1.5 years after the market launch.
March 2015

FIRST OPERATION IN SINGAPORE, THE GATEWAY TO THE ASIAN MARKET
Leading technology from Hannover is now also revolutionising the Asian market: first operation with MAGNEZIX®, a metal-absorbable implant, in the high-tech metropolis of Singapore. Leading doctors at the prestigious Tan Tok Seng Hospital convinced about the advantages of the revolutionary technology.
April 2015
GLOBAL DISTRIBUTION OF MAGNEZIX® SPEEDS UP
MAGNEZIX® has received the basic registration in Iran in record time, and is now also available in the United Kingdom, Chile and the United Arab Emirates.
May 2015
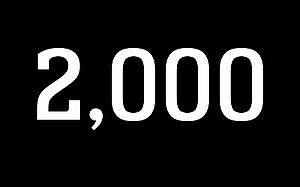
2,000 IMPLANTS
One year after receiving approval, MAGNEZIX® CS 3.2 implants have already been sold 2,000 times.
June 2015
![06/2015: Syntellix is Top-Innovator 2015 [] Top100 trophy](/fileadmin/_processed_/d/0/csm_1505_timeline_top100_b1b8e47556.jpg)
SYNTELLIX WINS "TOP-INNOVATOR 2015" AWARD
Syntellix is one of the most innovative companies amongst German medium-sized enterprises.
Success thanks to forward-looking corporate strategy: Syntellix AG wins an award as one of the most innovative medium-sized enterprises in Germany at the German Medium-sized Enterprises Summit in Essen on 26 June. - "Top 100" - mentor Ranga Yogeshwar awards the company from Hannover the - "Top 100" - label which has been awarded for more than 20 years. Before receiving the award, Syntellix was vetted during a two-stage scientific analysis process. In only its first year of participation, the company already leapt into the number 2 position in the group for companies with up to 50 employees.
July 2015
INTERIM ASSESSMENT AFTER TWO YEARS
This month boasted market launches in South Africa, Spain, Hong Kong and Macao. This means that only two years since it was launched, Syntellix products have now become available in 16 countries.
4000 implants have been sold so far internationally.
August 2015

MAGNEZIX® CS APPROVED IN A RANGE OF NEW SIZES
CE approval was issued for the additional diameters as part of the CS portfolio expansion in summer 2015. MAGNEZIX® CS screws are now available in four diameters and a total of 61 sizes. In addition, Syntellix is continuing its research on additional areas of application and use, and submitted a completely new product category to TÜV Rheinland for approval testing in the form of the MAGNEZIX® Pin, a bone pin available in four dimensions and 69 sizes.
September 2015

STATE APPROVAL FOR MAGNEZIX® IN SINGAPORE
Following the use of MAGNEZIX® implants in the Tan Tock Seng Hospital in Singapore since the end of March on the basis of a special authorisation, MAGNEZIX® has now received official approval from the prestigious state Health Science Authority (HSA).
January 2016
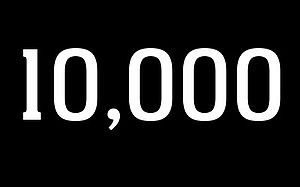
10,000 IMPLANTS
A new milestone: the 10,000th MAGNEZIX® implant sold.
February 2016

FIRST MAGNEZIX® SUPPLY CONTRACT
On the basis of an AOK co-operation agreement with the health centre at the ukb (accident & emergency hospital Berlin), patients covered by AOK medical insurance can now immediately benefit from MAGNEZIX® implants - all costs are completely borne by the health insurance company.
May 2016
PREMIERE: MAGNEZIX® IN ORAL SURGERY
The first jaw operation with MAGNEZIX® took place in May in the Clinic and Polyclinic for Oral and maxillofacial Surgery at the University Clinic in Dresden (www.uniklinikum-dresden.de).
The successful outcome of this first operation of its kind in the world confirms the versatility and reliability of implants made from MAGNEZIX®, and opens up a completely new spectrum of applications in oral and maxillofacial surgery.
June 2016

SYNTELLIX AG WINS THE TOP-INNOVATOR AWARD AGAIN
Syntellix AG is again amongst the TOP 100 this year after being assessed as part of a challenging scientific selection process looking at its innovation management and innovation success.
July 2016

NEW MAGNEZIX® PIN GAINS CE APPROVAL
From innovation to innovation: MAGNEZIX®, a globally unique material for simultaneously stable and degradable metal implants, is now also available as a CE-approved pin!
August 2016
![[Translate to EN:] 08/2016: 3-Jahresergebnisse sind hervorragend [] Bild 3-Jahresergebnisse](/fileadmin/_processed_/7/e/csm_1608_timeline_3jahre_7e0a82a58a.jpg)
MAGNEZIX® - CLINICAL FINDINGS AFTER 3 YEARS
The clinical findings of the three-year study with implants made of MAGNEZIX® demonstrate the special efficiency of this revolutionary technology. Patients involved were treated for a symptomatic Hallux valgus.
The findings revealed that the standard treatment with titanium implants and the use of MAGNEZIX® screws produced the same good results in both groups - but that the MAGNEZIX® implants have impressive advantages: the use of magnetic resonance tomography (MRT) in particular revealed that no metallic residues of the MAGNEZIX® screws were detectable after three years at the implant position. The MRT results also impressively demonstrated the bone remodelling with cortical tissue in parts.
November 2016

SYNTELLIX WINS GERMAN HEALTHCARE FUTURE PRIZE
Syntellix AG (together with HappyMed from Vienna) was awarded with the German Healthcare Future Prize by the Health Industry Club (cdgw) for its innovative medical products in Berlin on 10.11.2016.
December 2016
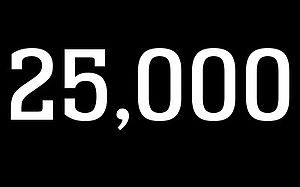
25,000 IMPLANTS
MAGNEZIX® continues to establish itself: with the 25,000th MAGNEZIX® implant, more implants were placed on the market within a period of 12 months than in the previous 2.5 years combined. No wonder, given the advantages magnesium implants offer over titanium- and polymer-based implants.
March 2017

NEW SUBSIDIARY
With Syntellix Asia in Singapore, Syntellix AG founds its first international subsidiary with a focus on the booming Asian healthcare sector. At the same time, the entry of Wintop Capital as the first international investor marks an important business milestone.
April 2017

AWARD: INDUSTRIAL PRICE 2017
Innovative medical device: MAGNEZIX® implants from Syntellix are among the leading solutions from over 1,000 applicants!
June 2017

INNOVATOR OF THE YEAR 2017
Syntellix is awarded a prestigious award as a highly professional, agile start-up company, which has succeeded in developing a world-wide novelty for surgery with enormous market potential, particularly through an impressive network and part management.
Jury: "The company's development could serve as template for a guide 'How to start a med tech start-up'."
November 2017

SYNTELLIX RECEIVES GERMAN MEDICAL AWARD 2017 FOR BEST PRODUCT INNOVATION 2017
The GERMAN MEDICAL AWARD, one of the few medical and management prizes to be awarded in Europe, honours pioneers in medicine with outstanding patient-oriented expertise and encourages them on their way to abandon conventional thought patterns and to advocate innovative, individual care for the benefit of patients in an ever more rapidly changing healthcare system.
December 2017

SYNTELLIX BECOMES INDUSTRY WINNER TECH IN STEP AWARD 2017
For the 12th time in a row, outstanding growth companies from Germany, Austria and Switzerland were honoured with the STEP Award. As a renowned company award for industries of the future, the award rewards the innovative strength of ambitious, emerging companies in the science, ICT and technology sectors and gives them valuable impulses for their further development. The STEP Award was initiated by F.A.Z. Fachverlag Frankfurt Business Media GmbH.
January 2018
SYNTELLIX CELEBRATES ITS 10TH ANNIVERSARY
On January 11, 2018, Syntellix celebrated its 10th birthday - and looked back on a decade full of innovations.
Febuary 2018
Clinical superiority2 over titanium: Comparative study on "Clinical superiority of MAGNEZIX®" published
A comparative study of clinical cure results after implantation of 100 MAGNEZIX® and 100 Titanium implants certified: No significant differences in stability and wound healing compared to titanium, great long-term cost reduction potential of MAGNEZIX® implants, no residual foreign material, and no longer necessary metal removal result in a clinical superiority of MAGNEZIX® compared to titanium implants ("clinically superior").
2 Klauser H. (2018): Internal fixation of three-dimensional distal metatarsal I osteotomies in the treatment of hallux valgus deformities using biodegradable magnesium screws in comparison to titanium screws. Foot and Ankle Surgery, published online, DOI: 10.1016/j.fas.2018.02.005.
April 2018

Successful registration of MAGNEZIX® CS and Pin in Hong Kong.
An important step has been taken: MAGNEZIX® receives the product registration in Hong Kong, besides Singapore another important hub for the market development in Asia.
Mayo 2018
MAGNEZIX® CS is approved in more than 50 countries.
The high number of internationally successful product approvals and registrations in various countries reflects both the high relevance for health management and the enormous development potential of MAGNEZIX®.
May 2018

Successful re-certification of the products for another 5 years.
Syntellix renews CE approval for the MAGNEZIX® CS range following a successful re-certification audit. A testimony to the high standards of the QM system of Syntellix AG.
July 2018

Syntellix wins the "Sustainability Award 2018" with the "Product of the year" award.
The "Business Intelligence Group" awarded MAGNEZIX® with the "Sustainability Product of the Year" award as part of the "Sustainability Awards program 2018". The Sustainability Award honors personalities, teams and companies that make sustainability an essential part of their mission and their business.
Maria Jimenez of the Business Intelligence Group explained the decision with these words: "It is clear to our judges that their vision and strategy will continue to deliver results towards a cleaner, more sustainable world."
July 2018

Successful admission to the funding program "German Accelerator Life Science".
Syntellix AG has been included in the coveted "Access" program of the German Accelerators Life Science (GALS) after a complex multi-stage selection process.
The German Accelerator Life Sciences is part of the funding program "German Accelerator" (www.germanaccelerator.com), initiated and sponsored by the Federal Ministry for Economic Affairs and Energy (BMWi). The aim of the German Accelerator Life Sciences is to assist and support German companies in the field of life sciences (including medical technology), which possess and demonstrate a high potential for innovation and implementation, in developing one of the largest domestic markets in the world. The program offers a comprehensive range of consulting and services tailored specifically to the healthcare market in the United States (including the Boston/Cambridge location), as well as versatile, valuable relationships with strategic partners, investors, multipliers and professionals.
September 2018

First scientific publication in Asia confirms the efficacy of MAGNEZIX® implants on Asian patients.
Choo et al. verify with the publication "Magnesium-based bioabsorbable screw fixation for hallux valgus surgery - A suitable alternative to metallic implants" to their pilot study in the internationally renowned journal "Foot and Ankle Surgery" (DOI: 10.1016 / j.fas.2018.09.001 ) demonstrably the function and efficacy of MAGNEZIX® implants in patients of Asian descent. An important "proof of concept" for the further development of Asia.
December 2018

Founding of subsidiaries Syntellix Philippines Inc. and Syntellix America Inc.
The preparatory work for the market launch in the USA leads to the logical foundation of a wholly-owned company Syntellix America Inc. - also in light of the company's excellent cooperation with the German Accelerator Life Sciences (GALS), which supports the market entry of Syntellix in the USA under the coveted Access program - on the world's most important market for orthopedic implants.
January 2019

MAGNEZIX® implants receive approvals for Indonesia, Bangladesh and Mexico
Syntellix has received extensive approval for its MAGNEZIX® implants for Mexico, the second most important market in North America. Thus, after Indonesia and Bangladesh, where a large number of registrations were recently achieved in the previous year, the approval of the highly innovative MAGNEZIX® products has now been achieved in a third market characterized by particular population growth and special population size. In view of the size of the population and the prospective patient potential, Indonesia and Mexico are currently two markets that are particularly important for Syntellix. In Indonesia, products have already been delivered and sales have been achieved, and the nationwide rollout is due to be held at a major congress in Solo City, Java, in April this year.
February 2019

Syntellix AG takes over 100% of MSE.
Syntellix AG has acquired 100 percent of the MSE Materials Science and Engineering Materials Center Clausthal GmbH. By taking over the long-term production and research partner, Syntellix will be able to design workflows even more efficiently, have more direct access to machine capacities and production control, as well as embed substantial expertise even more closely in the company and further expand its technology leadership. The world-renowned magnesium experts and former MSE owner Prof. Dr.-Ing. Volkmar Neubert and his son Volkmar-Dirk Neubert will remain with the Syntellix Group and the MSE team in their role as managing directors.
March 2019
Syntellix launches new product MAGNEZIX® StarFuse®.
Syntellix AG has announced the launch of its new MAGNEZIX® StarFuse® product range. The medtech company, which has hitherto mainly sold universally applicable implants such as compression screws and pins, is now launching a special implant for a strictly defined area of application on the market for the first time. Field of application for the StarFuse® implants are stiffening of the toe joints (PIP arthrodeses) for the operative correction of so-called hammer or claw toes, which frequently occur in connection with a hallux valgus (ball toe), which affects more than 10 million affected persons in Germany. With the new StarFuse® and other proven products from the MAGNEZIX® portfolio (CS, CBS and Pin), Syntellix now offers a system solution for foot surgery for the comprehensive treatment of malpositions with advanced magnesium implants.
March 2019

Founding of Syntellix China Pte. Ltd. in preparation for the planned market launch in China.
In China, arguably the most dynamic market from a global perspective, Syntellix has been able to reach an important regulatory milestone in the regulatory process, reflecting both the nationally-driven need and enormous interest in a highly innovative medical device from a health economics perspective. Both this progress and the need for near-market management of all other business activities led Syntellix to the earlier than expected Syntellix China, which is also registered and registered as a wholly owned subsidiary, but in some cases under Syntellix directorship, where appropriate with the Chinese minority Partners.
March 2019

MAGNEZIX® CSc 4.8 receives CE approval for Europe.
Syntellix AG has now also been granted Europe-wide approval for its next product novelty following the recent Europe-wide CE approval and successful launch of the new MAGNEZIX® StarFuse® product range. The new implant named MAGNEZIX® CSc 4.8 represents the by many surgeons long-awaited expansion of the proven implant portfolio of MAGNEZIX® CS screws. The CSc 4.8 has for the first time a newly developed magnesium surface ceramization, which is also completely bioabsorbable, and is available with larger diameters and longer dimensions up to 70 mm.
May 2019

Syntellix wins the German Innovation Award 2019 as "Gold Winner".
For the second time, the winners of the German Innovation Award were honored on May 28 at a festive gala event at the Technikmuseum in Berlin. The organizer of the Innovation Prize is the German Design Council, founded in 1953 on the initiative of the German Bundestag. Syntellix AG convinced the jury with its magnesium implant "MAGNEZIX® Pin" and was awarded the German Innovation Award in "Gold" in the category "Excellence in Business to Business - Medical Technologies". A "quantum leap in implantology" was the reasoning of the jury of the German Innovation Award for the presentation of the significant award to Syntellix.
With the German Innovation Award, the German Design Council honors pioneering innovations that have a lasting effect and offer added value for the user. In total, there were 695 innovation candidates, including industry giants such as Samsung, Bosch, Deutsche Telekom and the Swiss concern ABB - as well as hidden champions and start-ups.
August 2019

Syntellix ASIA and Chinese implant supplier Chunli agree on far-reaching collaboration.
A far-reaching cooperation between Syntellix Asia Pte. Ltd. in Singapore, subsidiary and Asia hub of Syntellix AG of Hanover, and China's leading orthopedic implant supplier Beijing Chunlizhengda Medical Instruments Co., Ltd has been agreed on.
In the future, it will accelerate the development of the almost limitless potential of the Chinese medical technology market.
September 2019
![[Translate to EN:] Syntellix Asia Tuas Einweihung [Translate to EN:] Syntellix Asia Tuas Einweihung](/fileadmin/_processed_/2/d/csm_190910_TUAS4_a05fa1f75d.jpg)
Syntellix ASIA opens production facility in Singapore.
Syntellix Asia Pte. Ltd. has visited Dr. Tuas Biomedical Park in Singapore in the presence of His Excellency, Ambassador of the Federal Republic of Germany in Singapore. Ulrich A. Sante, and Chairman of the Economic Development Board of Singapore (EDB), dr. Beh Swan Gin, the first international production site of Syntellix inaugurated.
The facility houses the world's first dry CNC machining for state-of-the-art magnesium alloys ever made in Asia. This will make Singapore a hub for the whole of Asia for the world's only bioabsorbable implants by German medical technology pioneer Syntellix AG.
The inauguration of the manufacturing facility for the world's leading medical device products in Tuas on September 10, 2019 marks a milestone in a year in which Syntellix has already seen groundbreaking developments.
September 2019

Outstanding media response also in South Africa after a Top Key Opinion Leader Meeting in Dublin.
MAGNEZIX® implants have already made their way in South Africa and are being used by six government hospitals. The use of MAGNEZIX® implants is particularly effective in public hospitals as it spares the costs of a second surgery for metal removal and facilitates recovery for patients who have to travel long distances for medical attention. According to the South African newspaper "The Citizen" "MAGNEZIX® could be the biggest disruptor in the surgical and orthopaedic industry in the last decade.”
September 2019

Establishment of Syntellix India Private Limited
In preparation for market launch in India, Syntellix founded its fifth subsidiary - Syntellix India Private Limited. In a joint approach with Indian partners, extensive work is being done prior to market entry, as the registration of MAGNEZIX® implants is moving forward. The use of MAGNEZIX® implants in India, as the second most populated country in the world, will bring considerable benefits for patients and saving potentials for the Indian health care system.
September 2019

World Patient Safety Day: Magnesium implants help avoid unnecessary surgery and risks
On September 17 2019, the 1st World Patient Safety Day was celebrated. The Day of Action is supported by the Patient Safety Action Alliance and World Health Organization (WHO), among others, and numerous campaigns and events are taking place throughout Germany and around the world. The World Patient Safety Day is intended to inform about effective solutions for more quality and safety in medical care and to sensitize the media and the public to central issues relating to patient safety.
According to the Robert Koch Institute (RKI), an estimated 400,000 to 600,000 patients are currently infected in hospitals in Germany every year. Of these, approximately 10,000 to 15,000 are fatal. The use of MAGNEZIX® implants eliminates the need for removal of material and completely avoids the risk of infection during a second operation and the corresponding in-patient stay. MAGNEZIX® implants thus make a significant contribution to patient safety.
September 2019

More than 20 scientific articles about MAGNEZIX® published
Following further publications, more than 20 scientific articles are now available demonstrating the efficiency, effectiveness, validity, safety and superiority of Syntellix products.
October 2019

Scientific article on the successful use of MAGNEZIX® implants in pediatric surgery
In the article "Magnesium-based implants in childhood and adolescence", well-known pediatric surgeons give an insight into their positive experiences and results after more than 50 successfully treated cases.
February 2020

MAGNEZIX® product lines "StarFuse®" and "Compression Screw 4.8 (CSc 4.8)" receive approval from the Malaysian authorities eight months ahead of schedule
Together with the already approved smaller CS variants, the MAGNEZIX® Pins and the cortical screw MAGNEZIX® CBS, the biomedical engineering, materials and life science company from Hanover now has a fully approved product portfolio in another Southeast Asian state.
February 2020

Granted approval for MAGNEZIX® CS and CBS in Myanmar
The two product families "MAGNEZIX® Compression Screw (CS)" and "MAGNEZIX® Cortical Bone Screw (CBS)" were granted initial approval in Myanmar half a year earlier than expected.
February 2020

EuroMinds visitors enthusiastic about MAGNEZIX® implants
With the title "The Future of Europe", the EuroMinds Economic Summit and the EuroMinds Knowledge Forum took place for the first time in Hamburg from 31 January to 1 February. Economic experts, scientists, politicians, media representatives as well as committed celebrities and also interested citizens were invited.
In particular, the topic "Medicine of Tomorrow" was lively discussed by the participants and followed with great interest. The corresponding panel was staffed by top-class experts: Together with Prof. Dr. Martin H. Kirschner from Syntellix AG, Ralf Möller, Hollywood actor and sportsman, and renowned doctors discussed the questions "how can technology be used for the benefit of patients" and "what role does technology play in the medicine of tomorrow?". Apart from the lectures and discussion rounds, interested guests were able to inform themselves in detail about Syntellix and the highly innovative and unique MAGNEZIX® magnesium implants - and thus experience "tomorrow's medicine" directly.
March 2020

Syntellix named a Winner of the 2020 Edison Awards
Since 1987, Edison Awards™ is an annual competition honoring excellence in new product and service development, human-centered design and innovation. MAGNEZIX® CSc 4.8 was chosen as a (silver) winner for the Medical/Dental category by a judging panel of leading business executives. This most recent innovation award further confirms Syntellix’s technology leadership and user orientation.
Among the nomination entries comprising the best products, services, and businesses in innovation for the year 2020, MAGNEZIX® CSc 4.8 was chosen as a winner by a panel of over 3,000 leading business executives in the fields of science, technology, design, engineering and marketing from around the world. “After a thorough review, the Edison Awards Judges recognize MAGNEZIX® as a game-changing innovation standing out among the best new products and services launched in their category,” said Frank Bonafilia, Executive Director of the Edison Awards.
March 2020
Start of the international MAGNEZIX® Webinar series
Since March 2020, Syntellix has also been offering various webinars in which renowned doctors report on their experiences and results with MAGNEZIX® implants. The topics range from hand and foot surgery to sports traumatology and pediatric surgery. 5 webinars are planned for the first half of 2020.
April 2020

26 international medical science publications about MAGNEZIX® products
There are now 26 international medical science publications that describe the advantageous properties or even the superiority of the highly innovative bio absorbable magnesium implants from the biomedical engineering, material & life science Company Syntellix compared to conventional titanium implants.
May 2020
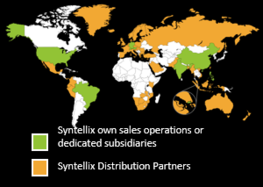
Syntellix now has product approvals in 66 countries around the world.
Both its own sales network and well-known partners worldwide ensure that MAGNEZIX® implants are now approved in 66 countries. Ascending trend!
October 2020

Syntellix wins the IMA Award of Excellence
In October 2020, Syntellix was honored by the International Magnesium Association (IMA) with the Award of Excellence in the "Wrought Product" category for the development of implants made from the magnesium alloy MAGNEZIX®.

