The world's first magnesium alloy approved for implants
A considerable number of orthopaedic implants had to be removed again in the past because they gave rise to undesirable reactions or had a negative physical or mental impact on patients.
The repeated operation is always associated with potential risks: damage to blood vessels or nerves can occur, and the anaesthetic and potential infections are a potential risk arising from surgery, not to mention the pain which patients have to endure, and the days off work (on average up to 3) because of reporting sick.
Modern medicine has long been searching for biocompatible implants, which after fulfilling their task are then resorbed by the body, and so do not unnecessarily stress patients or cause them problems in the long term. Scientists have long considered magnesium to be a very promising material for use in degradable bio-implants. The light metal is important for metabolism and bone growth, and is easily absorbed and degraded by the body. However, it is very reactive and difficult to machine.
With MAGNEZIX®, Syntellix AG finally achieved the long sought after innovative breakthrough in medical material research: and thus, the world's first magnesium-based material for metallically stable and simultaneously bioresorbable implants approved for use in humans became a reality.
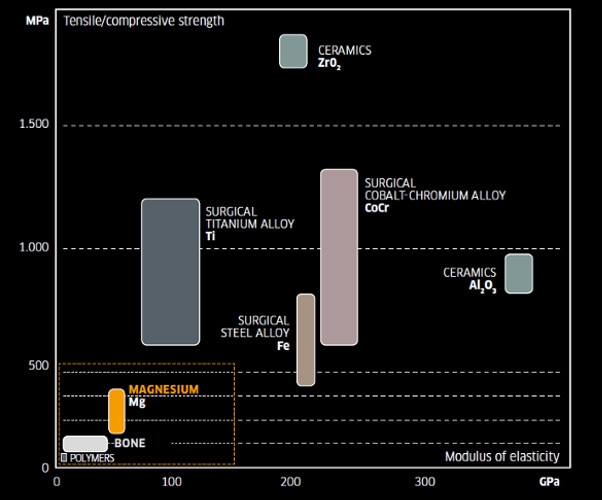
MAGNEZIX® [ma‘gnezi̯ks]: The alloy from the MgYREZr alloy system pursuant to DIN EN 1753 has a magnesium content of over 90 per cent, a grain size of less than 10 µm, and is manufactured using a so-called powder metallurgical process. This enables it to achieve a tensile strength which is much higher than that of the polymers (PLA/PGA), and lies in the range between bone and titanium.
MAGNEZIX® combines the advantages of two worlds
Today, titanium and steel are mainly used as non-resorbable metallic osteosynthesis materials. These metals have greater stiffness than bones, which can give rise to stress shielding. The inactivation of the bone metabolism in the fracture zone inhibits remodelling and can give rise to osteoporosis. Permanent implants made of titanium or steel also generate artefacts in radiological diagnosis methods, in particular in CTs. They are completely unsuitable for MRIs. In general, implants can give rise to foreign body reactions in the body, and cause allergies.
In the recent past, resorbable implant materials based on polymers (PLA/PGA) had mainly established themselves in surgical medicine. However, their use is associated with foreign body reactions in a large number of patients, which seriously questions their good compatibility. Because of the mechanical metrics which can be achieved, bioresorbable polymers can only be used in areas affected by low tensile, compressive and shear stresses - which strongly restricts the possible range of indications. Polymer implants are therefore hardly used in (partially) stressed areas for osteosynthesis. Current polymers are suitable for use in CTs and MRIs, but their visualisation is subject to limitations.
Materials made of magnesium in the form of various alloys have been the focus of general biomedical research since the turn of the millennium. And there are many good reasons for considering the use of magnesium in orthopaedic surgery: an implant made of this material would have a similar stability to a conventional metallic implant made of titanium or steel, but would also be capable of degrading. Magnesium-based implants have the additional advantage of being highly visible in CTs and MRTs, at the same time as only generating minor image artefacts.
Compared to the polymer materials already established in medical applications, resorbable implant materials based on magnesium boast good biocompatibility in-vitro as well as in-vivo. A significant advantage compared to polymer materials is the usually much higher strength of magnesium materials, such as the much more suitable ratio of mechanical strength and ductility for a large number of implant applications.
Conclusions: MAGNEZIX® is a magnesium alloy with stable metallic properties but which can be completely bioresorbed in the body and replaced by endogenous tissue. As a material for implants for osteosynthesis, this is an ideal combination of properties for providing temporary support.
MAGNEZIX® in comparison
Polymers
- resorbable
- degradation by hydrolysis
- pH acid (to 1)
- osteolyses (long-term)
- foreign body reactions known
- up to 6 years resorption
- inferior mechanical properties
Titanium and steel
- permanent, non-resorbable
- remain in the body until surgically removed
- non-osteoconductive
- stress shielding
- strongly marked artefacts in part in imaging diagnostic methods
MAGNEZIX®
- biotransformable
- degradation via corrosion
- pH alkaline (up to 9.5)
- active bone transformation zone: appears temporarily in part in X-rays as a resorption fringe
- osteoconductive
- infection inhibiting
- 1 to 1.5 years absorption, favourable (bone-like) mechanical properties
Bone-like and good compatibility - optimal for bone healing
The biomechanical properties of MAGNEZIX® are very similar to those of human bone, a fact which promotes the course of healing. The more favourable stress-expansion ratio (E-modulus) also counteracts the "stress shielding effect" which can occur when using conventional non-elastic metal implants.
An implant made of MAGNEZIX® has a comparable stability to a conventional metallic implant made of titanium or steel. However, it is completely resorbable - similar to other bioresorbable implants currently on the market. No allergies are known for the components of the alloy. Quite the reverse in fact, the extraordinarily good biocompatibility/suitability of magnesium is primarily a result of the relatively high daily requirement for this element by humans. The recommended daily intake of magnesium lies between 375 mg and 500 mg, depending on age and sex. Magnesium is not firmly bound within the bone, a fact which makes it very readily available. This means that a magnesium implant which degrades in a bone can also be considered as a source of the essential magnesium ions.
This combination of properties makes it much superior to the previously used polymer-based resorbable implants - so-called sugar screws - as well as permanent implant materials.
Osteoconductive and infection inhibiting - MAGNEZIX®, the better implant
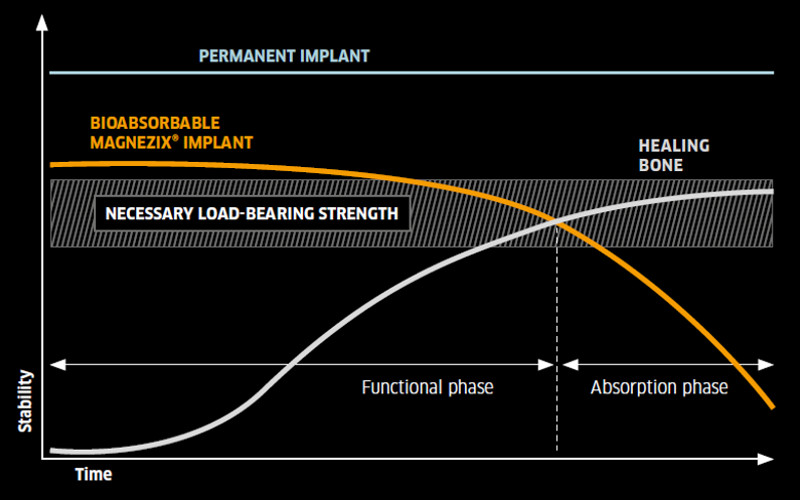
Heal first, then resorb - the MAGNEZIX® principle: Compared to polymers, MAGNEZIX® implants are not only degraded but transformed into endogenous tissue - and they stimulate bone growth.
Magnesium degrades in the body as a result of a corrosion process. This gives rise to an alkaline environment in the direct vicinity of the implant which inhibits bacterial growth. Of particular benefit for the human organism, are the hydrogen ions which are also released as a result of the corrosion process, and which have a special cell and tissue protecting function. In this context, hydrogen acts as an anti-oxidation material which binds directly to DNA-mutating hydroxyl radicals and peroxy nitrides, and renders them harmless. These positive properties of pure magnesium can also be expected from MAGNEZIX® (magnesium proportion of more than 90 %). Moreover, all MAGNEZIX® implants are packaged separately under sterile conditions to reduce the infection risk even further.
Magnesium is a biologically active material which can promote the healing process. In vitro as well as in vivo studies have demonstrated outstanding cell compatibility and excellent osteoconductive properties. In vitro tests not only revealed the high proliferation of human osteoblasts, but also a stimulation of their vitality. Moreover, bone regeneration (osteoid) was histologically confirmed on the surface of the degraded implant.
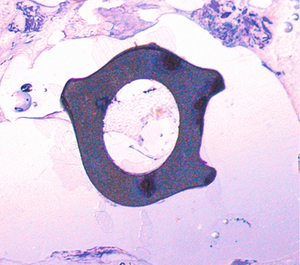
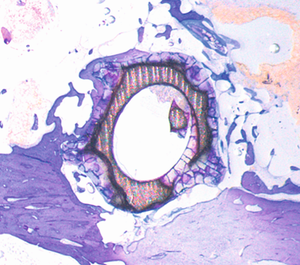
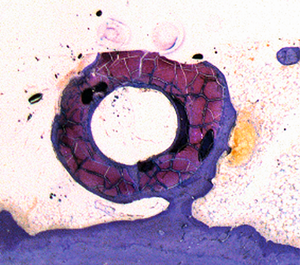
Transformation process of a MAGNEZIX® implant
The histological photos show the transformation process in the rabbit animal model. This shows a cross-section of a hollow compression screw at various times post-op.
Progress demands new thinking
The launch of an innovative medical product not only opens up a broader spectrum of new opportunities, but also always has to deal with initial resistance and prejudice. Innovations bring changes, and they must be convincing, they must be successfully tried and tested - but they also require that people break out of old patterns of thinking and behaviour.
MAGNEZIX®implants are innovative and completely unique world-wide. They are metallic and biotransformable, which means that they are resorbed by the body (and replaced in the process by bone tissue). This process takes place slowly and assumes that the implant changes and adapts inside the body. In individual cases, this means that temporary radiologically-visible bright spots can be observed around the implant. This phenomenon of "radiolucence" is a typical side effect of the degradation of magnesium, and therefore also of MAGNEZIX®: during the break-down process, the material naturally loses mass and weight. In addition, magnesium slowly releases small quantities of hydrogen during the degradation process which is absorbed over time. Because of the osteoconductive properties of MAGNEZIX®, osteoclasts and osteoblasts develop which are responsible for rebuilding bone, and osteoid is formed (bone matrix which has not yet been mineralised).
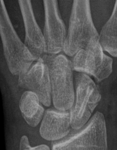
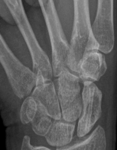
These active bone remodelling zones are completely unproblematic and should not be misinterpreted as a sign that the implant is coming loose. Although clearly visible, the described phenomenon of the radiolucent zones around the implant are only of a temporary nature, have no impact at all on bone healing, and disappear automatically. The findings from laboratory tests, animal studies and clinical applications, confirm that MAGNEZIX® screws are bioabsorbed within a period of approximately 12 months. After three years at the latest, the former implant is replaced by endogenous material which is almost identical with endogenic bone tissue.
It is recommended that the phenomenon of radiologically visible bright spots be recorded in the operation report/doctor's letter so that the persons subsequently treating the patient are informed about this special radiological feature of the course of healing.
Because MAGNEZIX® implants are completely degraded over time in the body, and therefore replaced by endogenous tissue, there is no need to remove the implant again in principle.
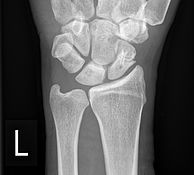
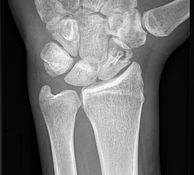
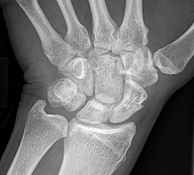
Bone remodelling zone shown in an example of a scaphoid fracture
MAGNEZIX® implants were developed to achieve a primary stability similar to steel and titanium screws, and a strength of up to 5 times higher than pins made on the basis of polymers. During the healing and degradation process, magnesium implants naturally lose their original shape over time. In some cases, they even appear to be "broken" in imaging diagnostic methods. This phenomenon has nothing to do with any deficits in primary stability but rather the fact that they are designed to deliberately degrade, at the same time as the healing bone regains the capacity to bear higher loads.
The fact that a MAGNEZIX® CS allows a self-cutting - not self-drilling - screw to be used, should also be borne in mind. In rare cases, the head of the screw broke because a pilot hole was not drilled in spongy and/or cortical bone. Because of the materials involved, drilling a pilot hole matching the selected screw length is essential for self-cutting implants to simplify the tightening of the screw and to reduce the rotation of small bone fragments.